EN14683 Medical Facial Masks BFE Test
EN14683 Medical Facial Masks BFE Test, describes testing methods for medical facial masks intended to limit the transmission of infectious agents between patients and clinical staff during surgeries and other medical contexts with similar requirements.
How to perform all the required steps to the Bacterial Filtration Efficiency Test (BFE) % following the EN14683 norm.
- Place the liquid (solution or suspension) to be sprayed in the Aerosol Generator;
- Prepare the Multi-stage Impactor and Connect the Pneumatic Line;
- Connect the Aerosol Chamber to the Impactor;
- Place the Extraction Condenser of the Impactor;
- Connect the Electronic Flow Control Sampler and check the Sampling Flow at 28.3 l/min;
Bioaerosol complete sampling system
Bioaerosol is a component of Particulate Matter (PM) in the atmosphere, and consists of airborne particles that have a biological origin. Bioaerosol has a very varied composition, including:
- Microorganisms (viruses, bacteria, fungi and their spores, algae, protozoa);
- Pollen;
- Fragments of animals, insects, plants;
- Derived substances (e.g. toxins and allergens) produced by any living species.

Cascade Impactor of the BFE Test Kit
The study of bioaerosol has numerous and different fields of application (allergology, industrial aerobiology, cultural heritage, bioclimatology, chemistry and atmospheric physics, ecology, epidemiology, biological pollution, mycology, microbiology, plant pathology, biological terrorism, indoor/outdoor air quality).
EN 14683 describes testing methods for medical facial masks intended to limit the transmission of infectious agents between patients and clinical staff during surgeries and other medical contexts with similar requirements.
Bioaerosol
The study of the microbial content of air has become increasingly significant in recent years when the need for “contamination-free” environments has become more evident.
Bioaerosol includes several types of primary bioaerosol particles (PBAP primary biological aerosol particles), with diameter ranging from a few nanometers (viruses), some micrometers (e.g. bacteria, pollen), >10-100 micrometers (e.g. fungi and spores), which are found in atmospheric particulates.
Bioaerosol: Bacteria, Mushrooms, Pollen, Virus
Knowing the dimensional distribution of bioaerosol allows to evaluate its aerodynamic behavior in the atmosphere (time of residence in the air, transport phenomena and deposition) and the potential health effects (deposition in different sections of the respiratory system).

Pollen Bioaerosol
Bioaerosol is sampled according to size by multi-stage impactors.
Bioaerosol is collected on an impact surface, consisting of a membrane, a fattened saucer or culture soil, and is studied using specific analysis techniques (microscope analysis; laboratory analysis for immunological, biological and chemical tests; culture-type techniques for arable life cells).
EN14683 BFE Test Applications:

BFE Mask Test by “La Sexta” Spanish Report Channel.
Research Team takes to analyze the three types of masks to know the level of protection according to their use. The first: a Chinese KN95 filter mask with 21 hours of wear. This class of masks protects around 95%. In other words: “Only 5% of particles that were in the air could be able to penetrate through the mask”, explain the scientists who work at the Paterna Technology Park.
In the case of the KN95 mask with 21 hours of use, the study has observed that the penetration of particles “exceeds that 5%”. It is almost 30%: “This may be due to the use that may have been given, the fact of washing or having used it for several days.
The second sample: a reusable hygienic cloth mask worn for 120 hours. It is the mask of a worker who wears it eight hours a day for 15 days. He assures that he has washed it 15 times. Is it effective as you are using it? We do the test again.
Although a reusable hygienic mask has a filtration capacity of 95% when new, in this case, experts say, “what we have found after these days of use is a filtration around 70%”. And remember: this type of mask can have “five wash cycles at about 60 degrees in a home washing machine.”
The third sample: a non-reusable hygienic mask from a user who claims to have worn it for an hour and a half a day for a month. To see if it is still effective, create a current of air similar to breathing. The test concludes that “the bacterial filtration efficiency is 87.76%”. That is, it is below the 95% required by the UNE0064 standard “
BFE BioKIT EN 14683 (Annex B: BFE Test)

KIT EN 14683 Electronic Flow Control Sampler
Electronic Sampler
- Auto-flow adjustment;
- Range from 0.5 to 70 l/min;
- Versions: Basic or X-Plus;
- Pump with Hepa filter.

KIT EN14683 Aerosol Chamber
Aerosol Chamber
- Pyrex tempered glass, dimensions 600 mm x 80 mm Ø;
- Upper flange of PTFE (HEPA filter included);
- Lower flange of PTFE (included: mask sample holder);
- Connections to: aerosol generator, 6 stage impactors and n° 4 additional side connectors (i.e. for optical particle counter: optional).

KIT 14683 Extraction Condenser
Extraction condenser
- PYREX glass Condenser;
- PYREX glass Condensation vessel.

KIT 14683 6 Stage Impactor
6 Stage Impactor
- Principle of inertial impact;
- Workflow 28.3 l/min;
- Direct sampling on Petri plates (90mm);
- Made of anti-corrant material;

KIT 14683 Particle Nebulyzer
Particle Nebulyzer
- Generating Aerosol from all kinds of liquids, suspensions and solutions;
- Integrated pump (no compressed air required);
- Adjustable air (dry) flow spray and dilution;
TCR Tecora offers the complete instrumental solution to perform the BFE (Bacterial Filtration Efficiency) Test, the BFE BioKit (Click to see) which also allows to perform the Breathability Test (differential pressure) (EN 14683 Annex C; ISO EN 9237).
To download the Datasheet, click here!
For more information about the BFE BioKit, please contact us:

 TCR Tecora
TCR Tecora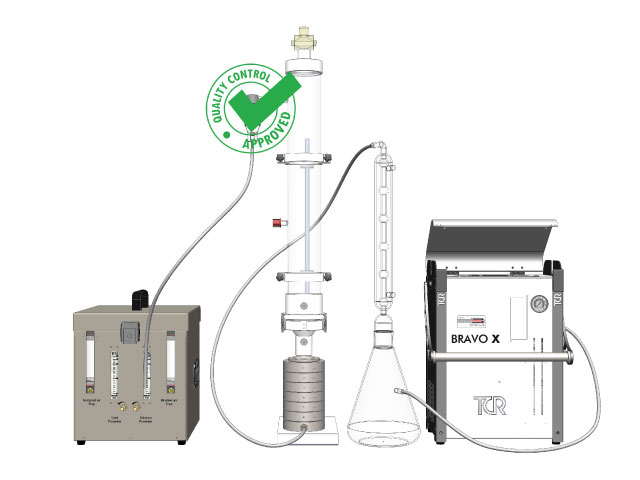


 TCR Tecora®
TCR Tecora®  TCR Tecora
TCR Tecora TCR Tecora
TCR Tecora TCR Tecora
TCR Tecora TCR Tecora
TCR Tecora

 TCR Tecora
TCR Tecora